[NOTE: This is still in development, and it is NOT YET WORKING. Since this blog post was written we have done more work on this idea, and so the diagram above and the post are now out-of-date. For the latest on everything see our Github repository for the project.]
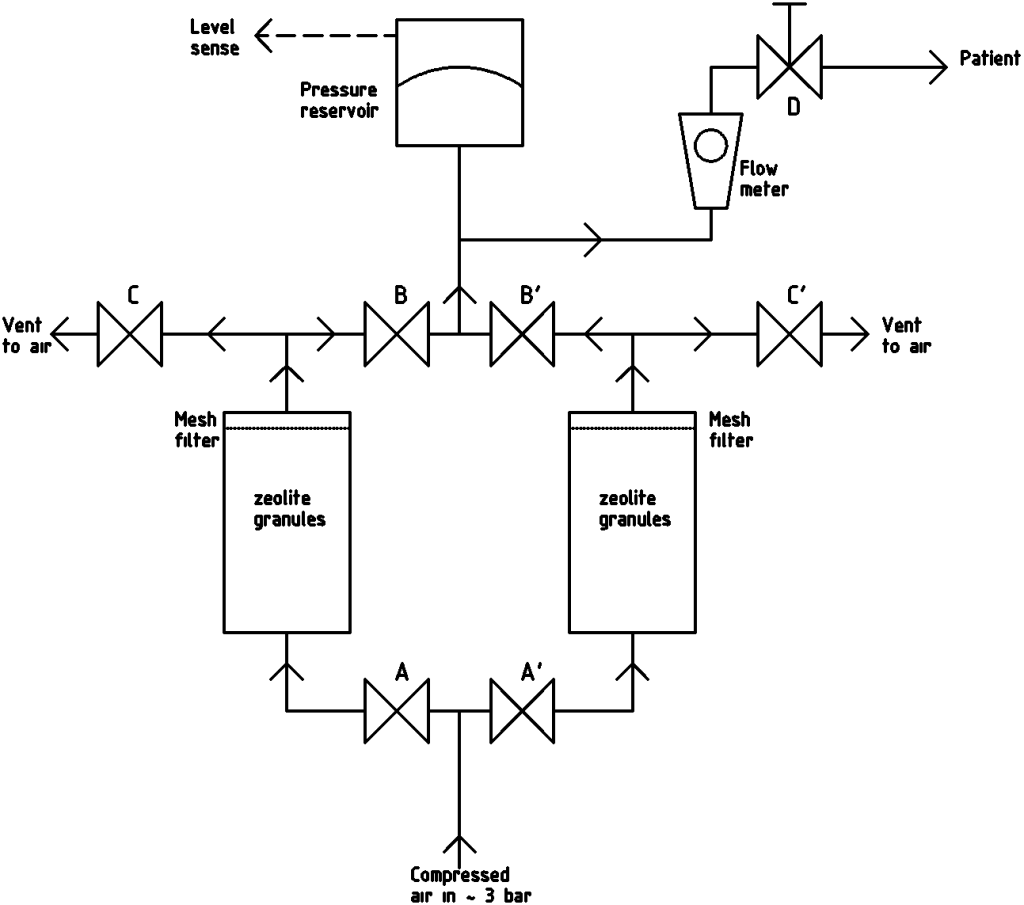
With the World’s current problems caused by covid-19, it seemes to us that an open-source oxygen concentrator would be a useful thing to have. These are fairly simple devices that work by pressure swing adsorbtion. The block diagram for what we propose is shown above.
(This article now incorporates some ideas from the comments below to make the information more easy to assimilate. A big Thank You to all who have made suggestions; please make more!)
To understand what’s going on, start at the end with the flow to the patient (top right). There’s a small manual thumb valve (D) and flow meter like this one that controls the flow of O2 concentrate (that is, up to 90% O2 with the rest N2 and so on from the air). 90% is the figure quoted for the commercial machines, and it’s rather confusing. We don’t think it means a gas that’s 90% O2 (which we’re pretty sure would be toxic); we think it means 1.9 times as much oxygen as usual; in other words a gas that’s about 40% O2 and 60% N2 etc. If you’re an anesthetist and actually know, drop a comment below, ideally with a reference.
The O2 is stored in a membrane pressure reservoir that has a sensor to tell the microcontroller (not shown) operating the entire machine when the level is getting low. That microcontroller would only need to be an Arduino Uno, or similar. The reservoir and sensor could literally be as simple an object as a plastic bag between two hinged flat sheets with a weight on top and a switch activated as the weight moves upwards (thanks@Joel_Driver; and see below).
When the pressure drops the microcontroller drives a MOSFET to open the solenoid valve A (all the valves shown are normally closed) and compressed air flows into the zeolite granules in the chamber on the left. N2 has a large quadrupole moment (an aspect of the pattern of electric charges round the molecule) relative to O2. This means that it “fits” into the surface of zeolite much better and adsorbs onto it in preference, leaving the O2 free in the chamber under pressure. After about 3 seconds valve A is closed and valve B is opened allowing that O2 into the pressure reservoir, then B is closed.
Then that process is repeated with the zeolite on the right, while valve C on the left is opened to allow the N2 in the left-hand chamber to desorb and vent to the air.
The best zeolites to use for this seem to be 5A or 13X, but we are still investigating that.
The compressed air would be required at the sorts of pressures and flow rates easily achievable by a 12 volt care tyre inflator. All the plumbing would be done using push-fit pneumatic connectors and PU pipe. The air would have to be dry, and have most of the CO2 scrubbed out of it. Extra tanks with silical gel and activated carbon should be able to achieve that. The carbon would be purged of adsorbed CO2 from time to time in the same way as the purge described above. The silical gel and zeolite would have to be dried periodically. The simplest way to do that would probably be to put them in a kitchen oven (electric, not gas…). You would have one set working in the machine, and another set being dried, then swap them over.
@Joel_Driver has suggested that 0.5L sparkling drinks bottles might work well as the zeolite pressure vessels. An alternative might be refillable in-line filter housing reverse osmosis units, which usefully come with 6mm push-fit couplings on the ends and built-in filters.
The mesh filters (cut from a kitchen sieve?) are to prevent the zeolite granules getting into the valves (or the patient…). It would probably be a good idea if the device was actually arranged as shown with the flow up from the bottom to the top for the same reason. As to size – the whole thing would be about shoe-box size plus the compressor.
Throughout this we have deliberately linked to non-medical-grade common very-low-cost components and materials of about the quality that one might expect in – say – a child’s swimming snorkel and mask. To be most useful, this thing has to be manufacturable anywhere in the World at low cost. And you trust your child’s life to that snorkel and mask…
We have started a Github repository for this device here.
How can you help? Here’s how we see this developing:
- We’ll get our version working.
- Other people make copies and variations.
- We and others make measurements (O2 concentration, CO2 contamination etc).
- Fix what’s needed to get those right.
- Fix anything needed to get the machine useful
- In developing countries, and in the developed world in remote and emergency situations.
- In the developed world in hospital and clinic situations.
Note that we explicitly acknowledge that less rigorous standards are appropriate for emergencies than for well-controlled locations, and that that allows more benefit to be obtained than insisting on the highest standard everywhere.
The first design is neither final nor prototyped yet, but there is nothing to stop you taking what we have posted on Github and building it experimentally. But the point where we would appreciate people getting involved is Stage 2. onwards. In particular it’s important that others change the design to make improvements so that the best designs emerge from a large number of experiments (this is what happened with the RepRap Project and is the reason it was so successful).
Connect with us
Keep up to date on the latest RepRap Ltd news:


It is worth noting that tyre compressors usually those for 12v cigarette fittings are designed for 10 minute operating max, unsure of the cool down period, but not for continual use. Will keep watching this 🙂
Yes. This would be intermittent use (though I don’t know what the duty cycle would turn out to be; 6 L/minute of O2 implies 30 L/min of air). Also it would be possible to arrange cooling. But there are lots of air compressors available, not just car plug-in ones.
I want to say I find it comforting to see those who try to solve problems with the current situation in a practical way. This is fairly old now but as a Respiratory therapist I can answer your question about oxygen concentration. It is in fact 90 percent that leaves an oxygen concentrator. At this percentage it can be toxic in the sense it can do damage to the lung tissue over a matter of a few days or longer of 24 hour use. But you are generating one to six litres of 90 percent oxygen per minute. As a typical person or patient may breathe 12 breaths per minute and about a volume of about 500 cc of air they are not breathing continuously over that minute. The person will breathe their volume of breath or “tidal volume” over 1/2 a second. If you take your maximum flow of 6 litres/min. It is a flow of 100 ml or cc in one second or 50 ml in 1/2 second. So when you give a person this 90 percent oxygen with nasal prongs they are mixing this concentration with room air to take their breath. Thus reducing the oxygen concentration they are breathing in significantly. But between breaths the nasal cavity has a natural reservoir that fills up which adds to make a higher concentration of oxygen per breath. We typically give these measurements in the hospital with 100 percent oxygen coming from the wall with nasal prongs as 1-6 l/min as 24/28/32/36 and 40 percent oxygen breathed in for the average adult based on the flow given.
Thanks for that! That’s very useful technical information.
You could create a manual compressor as some areas may not have a means to power say a 12vdc car tyre compressor, something like a foot pump. If you need help designing anything or advice, please ask, i design for the pharma and chemical industry working for a research and manufacturing company in the North West.
Also keep in mind it should definitely be an oil-free compressor!
I think the ones for air brushing might be an (better) option?
Concerning the max. operating time, a solution could be to use multiple ones and some switching power sockets to use them round robin like if nothing better is available.
About the mesh filters: I think a part of a sieve might be a good option and doesn’t even require a 3D-printer.
Very interesting post! Let’s really hope we don’t need it!
Thanks for the sieve idea. And yes – it would be easy to swap between compressors to give each a rest. I agree about oil-free, or maybe incorporate a moisture trap.
I think you’re taking a pretty narrow approach to the applicability of A.M. to help solve this problem. You say, “The mesh filters (which are probably the only part needing to be 3D printed)…” If your basic approach is to use all OTS materials but for that mesh, I can tell you from experience, there are OTS meshes that could serve this purpose just fine.
I’m not sure what all could be done in polymer A.M.; it’s not my specialty. In metal 3DP, we could print the chambers, the valve manifolds, the valve housings, and probably turn 30+ parts into 3-5.
And I think you’re going to want to use an accumulator ahead of valve A for that type of pump. Appropriately sized and pressurized, maybe you could find a pre-fill PWM cycle that would keep the pump from overheating. It says it’s 35LPM, but I’m sure that’s at 0PSI and derates with increasing pressure. You’ll have a more or less 6LPM constant demand, so it might be possible to find a balance.
We need 30 lpm of air to get 6 lpm of O2. I’m not too bothered about maximising 3D printed parts at this stage, or even sure if it’s a good idea in comparison to using easily obtained standard items – for example using fizzy drink bottles as pressure vessels (they’ll easily do more than 3 bar).
I’m a 3-D printer user, and would love to see if what I do can help. Is there any of my materials that can be devoted to the production of this?
We’ll make a prototype and put it out there. It will definitely be in need of improvement, and we think that’s when others can make and improve it.
I know nothing about zeolite granules, but could you not use muslin cloth/old t-shirt to contain them?
Also do you need the level sensor? Surely the user can see the ballon is empty and switch the compressor on? (Since the user will see that the ballon has burst/come off?) Edit: I yes, I see – switching valves for adsorption/desorption. (Sorry, civil engineer – no real idea about these things!)
Possibly yes. The sort we intend to use are a few mm in size. Another alternative is the nylon mesh from a kitchen sieve. I think the machine has to operate autonomously without input from the patient, who may be incapacitated, or simply asleep.
I would use fish tank filter mesh to retain the zeolite.
https://www.petco.com/shop/en/petcostore/product/imagitarium-2-pack-filter-sponges-2441408?cm_mmc=PSH-_-GGL-_-CAL-_-PME-_-PET-_-AQU-_-0-_-PTC_P_SUP_LIA-GG_FY19_SBU05_Companion_Animal_BOPIS-_-Local_Inventory_Ads-_-0&kpid=go_6471950367_76332473423_380230977733_pla-324731645754_c&utm_config=tad0iunwp&utm_campaign=PTC_P_SUP_LIA-GG_FY19_SBU05_Companion_Animal_BOPIS&utm_source=google&gclid=Cj0KCQjw6sHzBRCbARIsAF8FMpWvrk9XV2I03thK2hwCWsd7K4z4wFloGD94s9X_Brftn4KZVZ837BoaApyvEALw_wcB
Yes – that would probably work.
You don’t actually need a sieve. You can just use two coaxial tubes and have the air counter flow, reversing direction at one of the end. Gravity will contain the zeolite. This way you’d also create a larger flow path, which is what you want for adsorption.
Yes – gravity would contain the granules. But the problem is any dust produced by their rubbing together. That needs to be kept out of the valves.
My zeolite arrived today and it does indeed produce dust from rubbing together. You don’t want that gumming up the o rings in the valves or the patient. Bubbling through water prior to reaching the patient would also remove even more dust…
If you need help in Arduino programming, just let me know. Wouldn’t it be enough to use a relay module instead of a transistor to activate the valves and other actuators?
Thanks. We’ll put the software in the repo when we have it; contributions then would be welcome.
I don’t see why one would use relays in preference to MOSFETS, which are smaller, more reliable, and cheaper.
Zeolite is important because it has structure of large pores on the microscopic level. These little holes are important because they catch molecules . In this case it catches nitrogen molecules. The similar absorbing principle has an activated carbon.
Yes – that’s about the size of it.
Assuming you can use activated carbon in place of the zeolite would this work as complete canister and media solution? https://www.amazon.com/Inline-Granulated-Activated-Carbon-Filter/dp/B00551QCYM
The pressure reservoir could easily be an expansion tank (a potable one if needed); readily available from any plumbers merchant or diy store.
As for the zeolite container, reverse osmosis resin containers would do the job perfectly, would just need to switch everything up to 1/4″ pipe or modify them to take 1/8″. Probably just easier to source the solenoids and tubing in 1/4″ since they seem to just as available.
https://www.amazon.co.uk/Finerfilters-Refillable-Housing-Reverse-Granular/dp/B01N7HTTLD
A pressure reservoir may be too high a pressure (about 1 bar) for the low pressure side of this. The RO resin containers are a good idea.
Adrian, great project. Curious to learn where I could help out.
Acute poisoning from too much oxygen won’t happen so soon. At higher than ambient pressures oxygen can cause acute poisoning. The Central Nervous System might have trouble with too much oxygen in the long run, but this assumes that you actually get the oxygen into the blood stream.
I think it makes sense to only use any oxygen enriched supply with feedback like patient monitoring (vitals, checking visually, etc.) in place.
Yes – Finger clamps for measuring O2 saturation are widely available. and cost about $12.
I found this group of people who are also creating an Oxygen Concentrator:
Website: https://www.projectopenair.org/
Google Drive with files: https://drive.google.com/drive/folders/1IS1OyGSm_M9yfGUIV-yd1-hIz1FD5UvA
It would probably make sense to collaborate.
Thanks – yes, we know about that one.
Hi, nice idea! 🙂
Two comments, based on my lab experience with environmental sensors calibration
( https://stc.fs.cvut.cz/history/2017/sbornik/papers/pdf/6605.pdf?_=1491774461 ):
Mind that the compressor has to be oil-free to not produce any fine oil mist that would clog the zeolite and/or silica gel pores.
Also, the cycling realized with electropneumatic valves will cause pressure spikes and hence flow and concentration spikes throughout the system. The pressure reservoir should be large enough and also pressure regulator should be used to integrate these fluctuations.
Thumbs up,
Mike
Yes – we probably need dry air as input, too. We’ll see if a constant-force reservoir will integrate the spikes without a regulator first. Only add things when you know they are needed…
I have been reading papers on pressure swing adsorption… Lots of math and they all seem to assume a level of understanding/knowledge I do not currently have. Here are some questions based on what I think I understood from my reading.
1) It seems you can run lower pressure like 1.5 bar only loose <10% on the purity. Does anyone know if that is correct? I do understand that would also cut the output volume in half for a given canister size.
2) Do you need a quick drop in pressure with high flow to harvest the O2 before the N2 pops out of the media?
3) Is a purge with forced flow not required? vs only venting… hmm- I guess you would reach a steady state after a few cycles.
After writing this and it making me think more- I think these may all be purity questions.
These are really best answered by experiment. Sure – you can do calculations using the free Gibbs energy of adsorption and so on, and they will give you scales (like you can’t make a machine with 1g of zeolite…). But until you actually try it, you’re never sure what can be done. And experiment and evolve is often faster (and certainly more robust) than trying to design a perfect solution theoretically.
You could create a manual compressor as some areas may not have a means to power say a 12vdc car tyre compressor, something like a foot pump. If you need help designing anything or advice, please ask, i design for the pharma and chemical industry working for a research and manufacturing company in the North West.
Thanks for the offer! A manual compressor would be better than nothing, but it would either have to be operated by the patient (not ideal), or tie up a healthy person all the time. Might be better in those circumstances to make a bigger system that could create large volumes of oxygen for local distribution (weather balloons?…)
Interestingly enough, there might be some potential crossover from some of the Open Source ventilator efforts that mechanically squeeze a ventilator bag. (You wouldn’t have to use actual ventilator bags, just their functional equivalent.) If such a rig could provide the ~1.3 bar needed, it wouldn’t suffer from duty cycle issues and might produce dry/cool enough air to be a decent source. Harnessing one per zeolite vessel could provide quasi-continuous flow.
Outstanding project! This topic has been something I’ve been discussing on other forums. Some notes:
– Commercial (home) PSA Zeolite O2 Concentrators run ~$200-$400USD. A collection of valves, plumbing and controller is going to come to a sizable percentage of that price before adding a compressor and air filtration. My concern is that any sourcing issues that would impede buying commercial units would impede bulk sales of valves. Anyone have any ideas about the accessibility of commercial units?
– Gas rated components (which typically means nitrile/butyl or other alkane resistant components or gaskets, aren’t needed, but are there other considerations in working with high concentrations of O2? My experience is with welding O2, which I address with commercially acquired hoses/fittings/regulators/etc. Are hose clamp fittings appropriate for O2 at these pressures (which appear to be around 20PSI in the literature I’m reading. In gas work, pressures over .5PSI are ‘high pressure’)?
– I build multi-valve flame effects (booshbeats.com) and have recently, for ruggedization and simplification, switched to cheap 8ch relay boards and away from MOSFETs. The ability to use arduino-level signals, the inclusion of flyback and optoisolation, the low cost (<$8USD) and the ease of field replacement have won the day over solid state. These may be worth considering.
I'm eager to follow and prototype!
–Tim
Currently the parts have cost about £150. I would anticipate that reducing significantly with bulk buying. Plus I expect a considerable design simplification could be achieved over the first proptotype (which doesn’t even exist yet… 🙂 ), for example by using fewer, but multi-port, valves. Concentrated O2 is a bit dangerous, though at least it won’t catch fire… The current design will never have more than about 2 L in it at any time. Anything that will work in either mains water plumbing or with a normal workshop compressed air supply (5 – 6 bar) will easily withstand the pressures needed (around 3 bar). I don’t understand the use of relays though – they’re less reliable, less rugged, and more expensive than MOSFETs. And it’s easy to switch a MOSFET directly from an Arduino. See the draft circuit at https://github.com/RepRapLtd/Oxygen-concentrator/tree/master/Electronics/o2-controller (you’ll need the free and OS Kicad EDA – https://www.kicad-pcb.org/).
I am definitely swimming against the current with my switch to relays 🙂 I don’t want to over-emphasize their value, because every point you make about the preferability of MOSFETs is accurate. The challenge for me was, after years of using MOSFETs was that the points worked out to be true in detail, but failed in aggregate. Again, not trying to ignite a flame war, because I know I’m in the minority (and possibly just blindered into believing I made a valid choice.)
But here’s what led me to my choice. I needed a field replaceable unit for switching 8 12V solenoid valves (@ 2-3A each.) I’d made my own MOSFET based PCBs for this for a couple years but wanted something I could buy in smallish qtys. and didn’t need to solder. I started using pairs of 4ch MOSFET boards (example: https://www.aliexpress.com/item/4000300446132.html?spm=a2g0o.productlist.0.0.58b5298bssrsmy&algo_pvid=a0a98a17-f143-48b0-aac8-7a71da9fd44c&algo_expid=a0a98a17-f143-48b0-aac8-7a71da9fd44c-7&btsid=0ab6d67915847313380818239e4674&ws_ab_test=searchweb0_0,searchweb201602_,searchweb201603_) because I couldn’t find 8ch boards reasonably available. The build quality was shite (half of the boards were trash.) They were terribly static sensitive. And it was basically impossible to find a mate to those plugs.
For half the price of a 4ch MOSFET board, I could get 8ch relay boards (example: https://www.aliexpress.com/item/32983531022.html?spm=a2g0o.productlist.0.0.67df58c7h2h3uD&algo_pvid=dda25ef0-a019-4c9a-a2c3-2abd28d11ec6&algo_expid=dda25ef0-a019-4c9a-a2c3-2abd28d11ec6-8&btsid=0ab6d69515847315120014130e32cb&ws_ab_test=searchweb0_0,searchweb201602_,searchweb201603_) that were full of nice grace notes like active high/active low switching.
They make noise, which has actually been great for _my_ purposes (others may hate this aspect), they’ve been pretty rugged (far fewer fails than the MOSFET boards.) The NO/NC options for the outputs are super handy. They’ll switch the high side (which is a hassle with many MOSFET options.) And the 12V boards work fine with 5V arduino signals.
The other makers point and laugh at me, but I can replace them very easily in my controller rig when needed (rare,) and afford to keep a stock on hand.
I can’t imagine that I’d design a new product with custom PCB layout that used a bunch of relays like this. I’d almost for sure use MOSFETs, but for homebuilt devices, they’ve turned out to be a flexible, cheap, rugged solution.
(but please don’t take my explanation for an argument for using them.)
–t
Hi. First thank you so much for sharing this with the world! <3
I'd like to indicate you another open hardward project that seems related to yours. Maybe this would make sense you join effort?
"OxyGEN, is a device that responds to the lack of emergency respirators in the COVID-19 health crisis.
OxyGEN is an open hardware prototype that has been collaboratively developed by a group of engineers led by Barcelona based company, PROTOFY.xyz. Initiated March 2020."
https ://www.oxygen.protofy.xyz
The oxygen concentrator could easily be designed from three syringes, zeolite granules, a geared motor, valves and a controller. One should not dismiss people who offer their help!
The syringes would have to be quite large to get the volume required, but that might work.
(And no one has been dismissed by anyone, as far as I can see.)
Its needs a co2 scrubber, condensate control and a rehydration tank. The gas would have dangerously high co2 maybe 4% and at this level would be toxic, the gas would need to expel the condensed water and then rehydrate the enriched stream as it would be bone dry and not suitable for humans. It needs some work but its an easy fix.
Usung active charcoal would be the best solution. Two small separate tank that can be duty cycled. So, run it till its absorbed co2 to a set limit, then switch to the fresh tank, then blow off the saturated tank with comp air. Bubble the clean air stream through a container of clean deionised water. Replace the water routinely or add a uv light to keep the container sanitary cycle every 5 hours, Add a 0.2 micron gas filter to be 100% sure the gas stream is sanitary.
Add a heating element to the zeolite, you would need to cook off the water or replace it routinely. It might work better with a membrane filter but they are expensive.
You’re right. One of the problems with putting up a brief blog post and then going away to make the device is that you can either make it, or add details, but not both… We are going to use silica gel to remove water, and were maybe going to use Ca(OH)₂ to remove the CO₂. Your active charcoal would probably be a better solution, though. As you say, just bubbling the O2 through water should rehydrate it (though SCUBA divers breathe completely dry air without harm; they are healthy people, however…). UV LEDs at about 270 nm work quite well to sterilise water. To remove the water from the zeolite we were simply going to run it through a domestic oven.
What quantity or weight(Kg) of 13X Zeolite is required in each cannister to produce oxygen at 6 lpm ?
Also have you guys found success using plastic RO filter housing as pressure chambers? I doubt they might develop air leaks since they are designed for water and not pressurized air. Not sure about O-rings and stuff in it but do they work as zeolite containers ?
We have RO containers for that, but haven’t tried them yet. They have 6mm push fit connectors on the ends though, which should be ideal. And they’re sealed with O rings, which’ll be as good with air as water. We’re still at the construction stage. As soon as we have a working prototype (a few days, if there are no hitches and we can get supplies) we’ll be able to make measurements.
What quantity or weight(Kg) of 13X Zeolite is required in each canister, to produce oxygen at 6 lpm ?
Also have you guys found success using plastic RO filter housing as pressure chambers? I doubt they might develop air leaks since they are designed for water and not pressurized air. Not sure about O-rings and stuff in it but do they work as zeolite containers ?
Has consideration been given to 3d printing the majority of the device? The printing process would need to be carefully controlled to ensure leaks do not develop between the layers, but PLA (and even ABS) plastic would both be able to withstand 1-3bar, without bulky walls. ABS has the advantage of being able to be acetone-smoothed, which would help to seal any minor leaks if needed. The advantage is that you would be able to integrate the valves directly into the print to avoid the cost of buying valves separately. Careful (but relatively simple) valve design would allow for the valves to automatically switch the chamber/s from filling to purging. This would allow the pressure swing to happen mechanically rather than having to buy electronics to regulate this process. This could save $10-20USD that would have been spent on electronics. Ultimately, I think it would be optimal to have as simple of an electronic circuit as possible; Hopefully we don’t even need to have a microcontroller for the unit.
You could look into integrating at least the pump housing into the printed part as well. Depending on the pump design, you may be able to print the whole pump as well (perhaps with the exception of o-rings for sealing). There have been a couple of gear pumps designed and printed in the past that are available on grabCAD/Thingiverse, though I do not know how well they have performed. You would likely need to print the gears and perhaps the pump housing as well out of a nylon or higher-end TPU filament (See Ninjaflex Armadillo) to reduce wear inside the pump and improve overall efficiency.
If a single 3d printed pump is not able to pressurize all the way to the required 1-3bar, or if higher pump efficiency is desired, we could look at using a 2-stage pump, where the 3d printed pump forms the first stage and then the higher pressure is handled a second, smaller unit, or by a COTS pump.
All that could be possible, but we want to get the first one out using standard widely-available parts as quickly as we can. Then people can make variations on it such as you describe. One of my design principles is to substitute software for electronics wherever possible, and to substitute electronics for mechanics wherever possible. That almost always reduces costs, increases reliability, and makes things more adjustable and versatile. I’ve lost count of the number of machines I’ve designed in which the performance could be radically improved AFTER they were working fine initially simply by doing something clever in software. But again, people can make mechanical control systems if they want, of course.
Perhaps a few suggestions on your valve placement… the exhaust valves to the bottom of the tanks, a cross feed at the top high end pressures, limited in flow to provide a high O2 down purge for the empty cycle – about 25% of the producing cylinder) and a cross flow pipe and valve at the low pressure end to begin pressurising the depleted tank with higher conc O2 prior to main pressurisation- a three step processes, with duty cycles controlled by software.
I’m building a similar project and I’ve based it upon the DeVilbiss concentrator – the tech manual is online, with cycle times and pressures- worth a read. Using a 4 way rotary valve (as in their design) would be great, but difficult to home engineer. I am using 12v solenoid valves – £10 each, 7 required, controlled by Arduino and optical relays and for main pressure a Hyundai compressor, with a filter / water remover and also water absorptive granules on the low pressure inlet. Inline 5 micron filters on each high pressure outlet is limit dust from the granules. My main missing component is an affordable O2 meter… best wishes!
Those are all really helpful suggestions – thanks! Rather than basing ours on one of the expensive machines we were going to get a low-cost one from China and do a tear-down. But mysteriously you can no longer obtain them for love nor money; I wonder why?
We like the rotary valve idea, but – as you say – they are expensive. For Version 2 we may see if we can 3D print one.
Also, again as you say, why are O2 meters so expensive? Perhaps we should do an open-source one of those…
Yes, not surprised that nothing is available to tear down! I don’t think the rotary valve would be much of a problem if one had access to a lathe – which I don’t- but if you do then nylon rod 50mm would be the ideal material. This is available. I am prototyping one, but getting the faces flat, square and fair to seal is a problem, which is why I switched to the solenoid valve alternative – but I expect there to be a problem with pneumatic ‘hammering’ due to the short open closing times of 20msec, so the zeolite may be overly shaken and form dust. I’m using fine stainless mesh to contain this, but…
One interesting point raised by a previous called is the inadvertent raising of CO2 levels in the product gas. It is an interesting and notable point, and I shall research the partial pressure indications for a patient – I don’t have an answer to it at the moment, but I note the commercial unit I studied did not employ scrubbers (that I can see) and oddly their literature stated O2 levels as high as 95% at best, therefore implying the atmospheric 0.4% to 4% is either not concentrated or is removed. I don’t know yet…
On a practical note, the removal of water from the compressed air is vital or the zeolite columns will fail, and the removal of dust from the delivery system is also vital for obvious reasons. Also please consider the adsorption profile for 5A zeolites – this appears to be optimised at 45 psig, but it may not be necessary to maintain such pressure given a scavenged feedback cycle as previously described.
To others looking at these posts who cannot gain access to the correct grade of zeolites – and I say this with some very considerable reticence, please experiment with cat litter. Yes, I’m sorry but that is correct. The grey whitish sharp rock lump types (not clay or paper based types) are a natural zeolite but their pore size is not defined and I have no idea whatsoever of the efficacy – but it is worth looking at.
Finally I would add a caution. It is highly unlikely any health system would adopt a home made item for public use – at least until extremis. However the knowledge gained in manufacturing such an item would be invaluable. If the worst comes to the worst, offer your skills and ingenuity to the medical technical staff. Also, you may be tempted to breathe the gas of your own machines. I am. However be aware that you place yourself at considerable risk in doing so. If it really hits the fan – you may be glad of it, but until then please be careful. All the best to you and I salute your efforts and ingenuity. Ian.
I am looking into using a modified cycle so that instead of zeolites use of activated carbon which adsorbs O2 more than N2, basically a Nitrogen concentrator but with modified cycle to optimize the exhaust that should be rich in oxygen. Also you can use test tubes with iron wool in the bottom with them inverted over water to measure discrete samples of air for O2 concentration, not ideal but should work if you don’t have an expensive O2 meter on hand. Zeolites are expensive and probably going to have the same issue getting zeolites as the china made concentrators where they are no longer available even though they say they had stock when you ordered……. Activated carbon can even be made at home with some care, significantly cheaper and easier to get than zeolites. I should have preliminary results to see if this activated carbon idea is practical in a week if all goes well and supplies arrive on time. Activated carbon version may not give as high of volume or high a % O2 but if it’s much cheaper, more available and still produces an airstream of significant volume and O2 concentration it may be useful to someone that is having issues getting enough oxygen.
Yes – all that sounds interesting. Please let us (and more importantly everyone) know if it works.
I’d thought of the wire wool idea, but dismissed it as too slow. I shall try it now and see if I was wrong (which I probably was…)
We had no problem obtaining zeolite, incidentally – £25 per Kg, ordered online and arrived next day. See the Bill of Materials in our Github repository for details.
Adrian;
If you were to heat the wool end of the test tube it should react much faster…… I will be trying this method for my testing.
I was also thinking about using a portion of one of those chemical hand warmer sacs as they seem to eat oxygen rapidly but I have not figured out how to easily use them in a test tube or graduated cylinder yet. I think they are composed of Iron powder and some sort of activator perhaps?
I should have initial test results soon to see what kind of % concentration I can get with the simple steam activated granular charcoal I activated last night.
Thanks for the note on the low cost Zeolite, that is much much better price and availability than I was able to find, I believe it may be an issue to get them to ship to me in Canada however.
My wire wool experiment took about a day. Slow, but still useful. I understand about warming the wool (though one has to be careful of warming the gas, expanding it, and so having it bubble out of the bottom of the tube…). I wondered about arranging to pass an electric current through it. I may try that if I get a chance.
High concentrations of O2 can be dangerous, so flow rate needs to be carefully controlled, as a rule of thumb, less than 2l/min mixed with air at ambient pressure, should be safe for just about anyone. A doctor may prescribe a higher dosage, but that is not for lay people to determine. Also, if incorporated in a CPAP type ventilator, the pressure at which the gas mixture is applied is critical, Too much pressure can cause lethal damage to the lungs. Lastly, do not try this on yourself, if you become incapacitated you cannot disconnect yourself from the device. If in doubt, get advice from a qualified scuba diver, they know most of the dangers.
Yes – our design incorporates a flow control. [And I am a qualified SCUBA diver… 🙂 ]
What is the flow specification to achieve 5L/m output of oxygen? Pressure of the Compressed air, volume of the Tank and amount of the Zeolite granules?
We don’t know yet as we’re only just at the point where we can test the complete system. In advance, I suspect that we may need bigger zeolite containers than the ones we have to get more than about 2 l per minute, but we will see. As soon as we have results we will blog them.
>90% is the figure quoted for the commercial machines, and it’s rather confusing. We don’t think it means a gas that’s 90% O2 (which we’re pretty sure would be toxic); we think it means 1.9 times as much oxygen as usual; in other words a gas that’s about 40% O2 and 60% N2 etc. If you’re an anesthetist and actually know, drop a comment below, ideally with a reference.
In my understanding, the 90% is a volume fraction measurment (i.e. absolute)
Here is a WHO publication that specifies as much (see section 2.2.1):
https://apps.who.int/iris/bitstream/handle/10665/199326/9789241509886_eng.pdf;sequence=1
This publication cites the ISO standard for Medical oxygen concentrators: https://www.iso.org/standard/59978.html (ISO standards are not freely available, I did not download the full text myself)
I also happen to know that medical O2 concentrators are repurposed in applications such as glass lampworking (where you mix O2 and Propate/natural gas to get a hot enough flame to melt borosilicate glass) This requires O2 to be relatively pure, and is more commonly done with tanked oxygen.
Note that it is 90% pure as it leaves the concentrator, in the lungs, it is mixed with air and is only ~30% oxygen by volume.
Thanks – that’s really helpful. I’ve downloaded the WHO spec and put a copy in our repository. As you say, the ISO document is behind a paywall, which seems particularly unhelpful and pointless.
I tried the O2 concentration test idea but used some of the powder from one of those hand warmer pouches instead of the iron wool.
It works fairly quickly so you need to fill the tube quickly or else you will throw off your results due to the reaction taking place and eating a ml or two over only a couple minutes at the start. It takes about 2 hours to eat up the 21% oxygen from air using about 1cubic centimeter of the powder wrapped in about a 2.5×2.5″ square of paper towel with then tied off as a small pouch. I put this in the bottom of a 100ml graduated cylinder then stuck a small N32 magnet on the outside bottom of the cylinder to hold the powder pouch in place. I actually made two pouches and used 2 cylinders, filled one with a test volume of air from the activated carbon system and one with just room air as a control. Used a small silicone hose to suck out the excess air in each to reach the 100ml mark then left them for a couple hours. It took about 2 hours to reach ~79ml for the control, which is in good agreement with the ~21% O2 that the air is supposed to have. I left for 3 hrs to make sure it had all been eaten by the iron powder mix. I appears you can use the same pouch at least twice for two different sample tests as it can eat much more than 20ml of oxygen if you keep it away from oxygen in between tests. Unfortunately my initial test with the activated carbon adsorbed air showed very little (maybe 1-2%) O2 enrichment, very close to the accuracy of the test. I will try some vapor deposition of carbon from Toluene vapor at high temp onto the act carbon to see if that improves the sensitivity to any significant level. If the home brew selective CMS proves ineffective I will order some Zeolites.
I am trying to figure out how much weight of zeolites I would need and what the chamber sizes will need to be if I end up using the zeolites.
What is the approximate weight of a liter of the zeolites everyone is using for these, has anyone calculated the approx density?
Sorry to hear that the carbon didn’t work.
Zeolites are not all that dense. But the density depends very much on the form, and how it packs. The stuff we have is little rods about 1.5mm in diameter and 5mm long. We haven’t checked volumes, but – pure guess – I’d say the density was about 1.5 g per ml.
Update on inadvertent CO2 concentration – a question raised by a previous contributor. I noted that the technical manual of a commercial concentrator made no mention of carbon dioxide removal, and It appears that 5A zeolite is already used to remove CO2 in NASA equipment https://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa.gov/19980237902.pdf
In response to the question of quantity of zeolite required, my research points to a kilogram per tank for production of 5 litres per minute which seems to fit with the berry approximate estimate of the commercial tank volumes. The tank volume will of course depend upon the volumetric displacement of the pellets.
On the cycle times, temperature, pressures and humidity. Again analysing a commercial tech manual they seem to use 20 seconds pressurising a tank to 30 psig (which surprised me as I expected 45psig) and bleeding to a 8.5psig storage tank. Again, I was surprised to see no moisture removal in the manual, but am reasonably sure that a dessicant stage is important to maintain zeolite function. Adsorption works better at lower gas temperatures, tailing off over 60 degrees, so consider this in compressor function.
Degassing the nitrogen – the above diagram seems to rely on degassing by depressurisation only. I suspect that this is workable, but suggest a top flush pressure purge with sacrificial oxygen rich gas, which may increase the purge efficiency, also a cross feed purge from the low pressure side for the same reasons.
On gas conc. the manual states better than 90% O2, but as a previous reporter said – is this absolute or relative to atmospheric oxygen? I’ll have a look at that one in between pressure testing today.
Best we have been able to find 90% means absolute – 0.9 volume fraction. It may be that the zeolite scrubs the CO2 as well as the nitrogen, which would be nice if true. But – as you say – moisture has to be a problem, particularly as manufacturers have no control over the RH wherever their machines are operating.
Its a really nice website. Thanks for giving full information about Oxygen Concentrator.
Thanks! We hope that it will help!
My design is now functional and producing reliable oxygen that will reignite a smouldering splint. Perhaps you would like to view my YouTube video… https://youtu.be/jhrS0ttp-mU
Best wishes, Ian
That’s brilliant! Have you put details (other than the video) online anywhere?
Not yet – seems a bit arrogant to assume I’ve done anything better than anyone else here, also despite it working well enough I’d like to make improvements to the cycle times, and venting flows so I’m not at all convinced my design is terrible worthy at this point. Thanks for your kind comments! I will share anything in the meantime if anyone is interested. Ian
Fair enough!
Thanks for doing a lot of the legwork. I have been fiddling with a DIY concentrator, but no real results yet. Used PVC 2 inch pressure pipe and lots of brass lab taps for gas flow. Used raw clinoptilolite grains from Amazon. Very cheap (about 1 $/lb), but didn’t seem to do anything. Don’t believe it contains the right form of zeolite (X13 geometry). Got some X13 on Ebay but have not got good results so far. Maybe need to dry out beads and make a water Absorber? I think clinoptilolite will act a good dessicator too instead of silica gel beads.
Had good success with measuring oxygen two ways. First was to use soda straws made of clear plastic. 1/2 inch ones are easiest. Fold over end and hot glue to make cheap vessel. Use steel wool, first soaked for a least a minute in full-strength vinegar with lots of salt added. Carefully dry the steel wool on a paper towel, otherwise water will plug up the wad and won’t work well. Should measure oxygen in about 1/2 hour. Other method uses two inexpensive syringes (60ml) with a glass tube between them coupled with 1/4 inch id tubing. Insert a wad of dry steel wool in the glass tube. I added a very small peace of match head to the middle of the wad that will ignite when held of a gas flame. Push the gas gently from side to side until no more gas absorbtion. Let cool and measure oxygen content.
We like the O2 concentration measuring devices. We have used a test tube with damp steel wool stuffed in the end suspended over a water bath, but that takes about a day to give a result. We have thought of focusing the sun through a lens on the wool, but the gas mustn’t get so hot that it bubbles out of the tube. We agree about drying the zeolite. We haven’t got good results yet and, despite having an initial silica gel drying chamber, we think that may be due to moisture adsorbing onto the zeolite. We may have to put it in the oven…
I just put the zeolite in the oven. Initial charge was about 600 grams, and got off about 60 grams of water after 4 hours at 500F (260C). Let it cool in turned off oven for 20 hours and it gained back 5 grams! Very good desiccant apparently. I also had some raw clinoptilolite zeolite, but not x13 beads, from Amazon, very cheap and will use it for a desiccant. Dried in oven and got about same amount of water driven off, and it also gained back a few grams after cooling in air. Don’t have any silica gel beads. I think this will work.
If you use the vinegar and salt solution with the steel wool, you will get results in a much shorter time. An hour or two. Be sure to mostly dry it off then.
If I get it working, I was thinking of using a compressor from a discarded fridge. The main advantage is it is quiet, not the noisy compressor that could drive you daft. Would have to use an filter to keep out the oil mist. It can also be used as a vacuum pump, and some of the PSA devises draw a vacuum during the evacuation phase. I’ll keep you posted. Regards, Dick
Thanks! Those are useful numbers to know. We will do a similar experiment on the zeolite in our machine (which optimistically at the moment we are assuming is dry because of the silica gel upstream of it…).
Sorry, that was “cool of in turned-off oven for two hours”
You can build your on DIY High Flow Oxygen Concentrator that produces 15 LPM at 97% concentrated oxygen using common parts found at local hardware stores at the OxiKit website. Step by step video instructions, part list, code for the arduino board and engineering design schematics are all listed.
Thanks! For those interested, here’s the link: https://oxikit.com/
Can you make a video mp4 to make it understand easily
At the moment we are having problems getting the zeolite to concentrate the oxygen. We will make a video when we have the bugs ironed out.
Its a good website. Thanks for giving full information about the Oxygen Concentrator. You can also check at hospitalbedindia.com
Hello i am working on an oxygen concentrator using my own design. While doing research i ran across you site and have reviewed what you are doing. From my research i am adding a venturi vacuum device for the purge cycle. in your design i would TEE the two Vent to Air pipes together to the vacuum port of a venturi Vacuum device then add another solenoid controlling compressed air through the Venturi vacuum device. Change the software so that during the purge cycle keep the air inlet solenoids closed This will result in removal of the concentrated Nitrogen in the Zeolite allowing the Zeolite to absorb more Nitrogen during each cycle. but it means that the tanks must be capable of handling the vacuum developed. Should not be a problem as most venturi vacuum devices develop about .89 bar or 13 PSI. Also they are not expensive.
Another point of interest is that under vacuum Water boils at very low temperatures. if you pull a vacuum close to the .92 bar that a venturi vacuum is capable of, the boiling temperature of water is about 37.5 C This would help keep the Zeolite dry.
That’s a very good idea. We have suspended this project temporarily for the simple reason that we couldn’t get it to work… We suspected that we need to heat the zeolite above 100 C before using it; blowing dry air through it doesn’t seem to get rid of the water. Your vacuum suggestion would be simpler if we were to incorporate it for getting rid of the nitrogen too.
When we get back to it we will certainly look at using a venturi vacuum as you descrribe. Thanks for the suggestion.
i suspect that the multi function valve that the commercial units use has a venturi system built in. since the commercial units are all self enclosed and everything is in on small unit i suspect that the compressed air temperature is high enough to keep the Zeolite warm enough that the water is pulled out of the Zeolite at all times. in my unit I am also going to use a Water separator (used for spray painting) and then run the air through color indicating Silca Gel in a clear tank to remove any remaining water before sending it to the Zeolite tanks. The air i am sending to the venturi vacuum will bypass the dehydrator setup as it doe not have to be dry. Also i intend on using the O2 for welding and need to compress the O2 to about 30 PSI minimum Still working on a cheap compressor that will work with compressed O2 As i don’t know if i can generate enough O2 fast enough to run the welding rig.
Pingback:Open Source Oxygen Concentrator Design – QDot2
Pingback:Open Source Oxygen Concentrator Design – Ama HUBS
Pingback:Open Supply Oxygen Concentrator Design | Electronics For You - ELECTRICIAN WORLD NEWS
Thanks for this knowledge sharing on oxygen concentrator. I am from India and we really and desperately in need of these to help more and more people.
I am struggling with the size (diameter and length) of the zeolite adsorbent column and the amount of zeolite to be added in to get 5 Ltr/min oxygen at 95% purity rate.
Kindly help.
What flow and purity are you getting? Your problem may be water in the zeolite. You can try drying it in an oven, letting it cool in a sealed container, then putting it in the machine. But note – this project is still in development; we have not tried that.
Hi Adrian.. We a group of engineers were try to make one oxygen concentrator. We are using Zeolite 13X. We tested our design with pressure inside sieve beds as 4 bar and holding time of 5 sec. However, we found that the O2 percentage is not going above 30%. We are unable to find out what is going wrong. Can you please suggest as per your experience.?
Thanks in advance.
We think the problem may be that the Zeolite has too much water in it. If you want to take this further, try drying the zeolite in an oven above 100 C, putting it in a sealed container, allowing it to cool, then loading it into the machine.
I want to know that if there is 3bar pressure built up in the Zeolite container, and waiting for 12-15s for adsorbtion, after releasing the gas to the oxygen concentration tank, all oxygen would pass into the cylinder after 20% reduction in the 3bar pressure? If oxygen is 20% in the air, at about 2.4bar pressure in the Zeolite container, all oxygen would have already passed to the oxygen tank? Am I correct? Please guide.
Secondly, if there is a leakage in the Zeolite container, and we lose some pressure to it, that would mean loss in oxygen only .. since Nitrogen is already adsorbed in zeolite. Am I correct?
No – it won’t work quite like that. Zeolites hold nitrogen in preference to oxygen and retain it. But they don’t do so perfectly. So some oxygen will enter the zeolite, and some nitrogen will remain outside.
This will also affect what will happen with leaks. They will mostly be oxygen, but also have some nitrogen in.
is someone there still trying….this project …i have question ..and advice about zeolithe…different type…compressors and electrovalve…please respond
We haven’t had time to work on this in the last few months, but we may revive it in the future. Most of it is working, but there are some problems remaining and it does not yet concentrate oxygen. By all means ask your question and we’ll do our best to answer it.
Hi
I bought from Germany zeolite 13x 3-5 mm …I would like to know if the size of the granules matters … and if so … if it can be corrected by weighing … a larger container or a higher pressure ?
Thk
I don’t know, but I imagine size would be important, as the smaller the particles for a given mass, the greater the surface area for diffusion in and out. Too small, however, and they would be difficult to retain behind filters. Most concentrators seem to work around 2 or 3 bar pressure, regardless of the particles they use, so I think higher pressure may not help.
Thank you for your answer
I searched for a month continuously in china and india LiX zeolite … the lowest price I found is $ 150 per kilogram … I read on another forum about a possibility to turn 13x into LiX zeolite with the help of batteries laptop 18650 … what do you know about this … and about the priorities … considering that on the world market the price of the raw material for oxygen concentrator has practically increased 10 times … wouldn’t it be a good idea to focus our time and money to build one… accessible to all?
It seems that Mr. Adrian rarely visits this form …
I check it every few weeks. It is one of 56 projects that I am currently working on.